About Insomnia

Understanding Insomnia
Insomnia Definition
American Psychiatric Association DSM 5 Criteria defines Insomnia as a condition in which people have difficulty falling asleep (DFA), difficulty staying asleep (DSA) and/or with early morning awakening (EMA).*
Insomnia Epidemiology
Symptoms occur in 33 to 50% of the adult population *,†
- As many as 95% of Americans have reported an episode of insomnia at least once in their lives Younger adults have more of a problem falling asleep*,†
- Middle-age and older adults complain of middle-of-the-night or early morning awakenings *,†
- Women are twice as likely to have insomnia than men *,†
- Around 40% of people with insomnia have a concurrent psychiatric issue *,†
* Dopp JM, Phillips BG. Sleep–Wake Disorders. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach, 10e New York, NY: McGraw-Hill
† Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(5 Suppl):S7-10.
America Insomnia Survey
-
American Insomnia Survey was a large national survey which included more than 10,000 adults from a commercial health plan.*
-
The America Insomnia Survey states*, that American adults are almost twice as likely to complain about DSA and EMA than DFA.
-
This survey found the most common symptom profiles in individuals with insomnia to be EMA only, DSA only, or both EMA and DSA.
- Approximately half (48.3%) of the respondents with insomnia complained about either two or three symptoms
* Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS). Sleep. 2011;34(8):997- 1011.
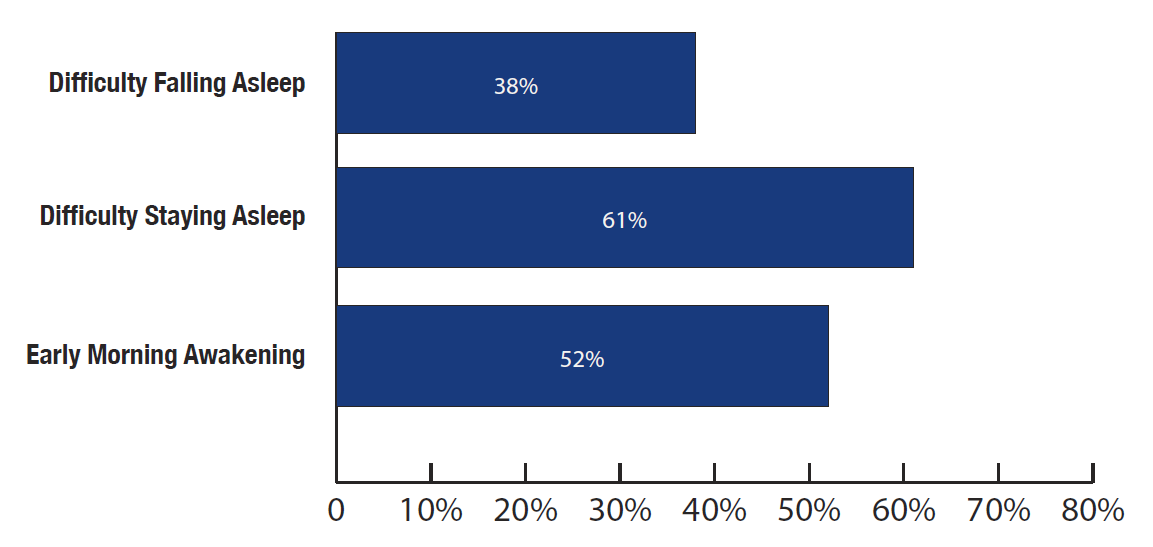
The American Insomnia survey found more respondents complaining about difficulty staying asleep (61%) and early morning awakening (52%) than difficulty falling asleep (38%).
Insomnia & Comorbidities
Insomnia & Depression*
Approximately 40% of adults with insomnia also have a coexisting psychiatric disorder, the most common being depression. Certain psychiatric conditions may be linked to Insomnia and can even be considered a diagnostic criteria. Psychological issues can lead to difficulty falling asleep, insomnia can lead to mood swings and shift in hormones. Studies suggest insomnia can trigger or worsen depression. The good news is that both conditions are treatable.
Insomnia & Anxiety*
Trouble falling asleep can cause someone to worry or be nervous. Certain anxiety symptoms that may lead to Insomnia include:
- Overthinking
- Excessive worry
- Tension
- Feeling overwhelmed
- Overstimulated
Anxiety may lead to problems with trouble falling asleep or maintenance of sleep throughout night. Once it starts happening on few occasions you may start feeling anxious and it triggers difficulty falling asleep. In patients comorbid with both, anxiety showed before insomnia in most instances. Various cognitive and mind-body techniques maybe helpful to people to facilitate falling asleep and relive anxiety.
Insomnia & Lifestyle†
Unhealthy lifestyle and irregular sleeping habits can create or initiate causes that may lead to insomnia. Some specific lifestyle factors may include:
- Working late in the evening & night
- Long naps in the afternoon
- Jet lag or Night worker
* Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(5 Suppl):S7-10.
† Lopes CS, Robaina JR, Rotenberg L. Epidemiology of insomnia: prevalence and risk factors In: Sahoo S, editor. Can’t sleep? Issues of being an insomniac. Rijeka: InTech; 2012. p. 3-22.
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS. See full prescribing information for complete boxed warning.
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required.
- Follow patients for signs and symptoms of respiratory depression and sedation (5.1, 7).
- The use of benzodiazepines, including DORAL®, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Before prescribing DORAL® and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (5.2).
- Abrupt discontinuation or rapid dosage reduction of DORAL® after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue DORAL® or reduce the dosage (2.3, 5.3).
CONTRAINDICATIONS
DORAL® is contraindicated in patients with known hypersensitivity to DORAL® or other benzodiazepines. Rare cases of angioedema involving the tongue, glottis or larynx have been reported in patients after taking the first or subsequent doses of DORAL®. Some patients have had additional symptoms such as dyspnea, throat closing, or nausea and vomiting that suggest anaphylaxis. Patients who develop such reactions should be treated in an emergency department and should not be rechallenged with DORAL®.
DORAL® is contraindicated in patients with established or suspected sleep apnea, or with pulmonary insufficiency.
WARNINGS AND PRECAUTIONS
CNS-Depressant Effects and Daytime Impairment: Doral® can produce CNS depressant effects, including daytime impairment. Patients should be cautioned against driving and other activities requiring complete mental alertness. Alcohol generally should not be used during treatment with DORAL®. Additive effects occur with concomitant use of other CNS depressants. There is an increased risk of next-day psychomotor impairment if higher than the recommended dose is taken, if co-administered with other CNS depressants, or if taken with less than a full night of sleep remaining (7-8 hours). The use of Doral® and concomitant CNS depressants may require downward dose adjustment and the concomitant use of Doral® with other sleep-hypnotics is not recommended.
Because DORAL® can cause drowsiness and a decreased level of consciousness, patients particularly the elderly, are at higher risk of falls.
Need to Evaluate for Co-morbid Diagnoses: If insomnia worsens or fails to remit after 7 to 10 days of treatment, this might be indication of an underlying illness that should be evaluated.
Abnormal Thinking and Behavior Changes: Abnormal thinking, behavior changes, and complex behaviors such as “sleep driving” (i.e., driving while not fully awake, with amnesia for the event) and other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic. As with sleep-driving, patients usually do not remember these events. Some of these changes include decreased inhibition (e.g., aggressiveness and extroversion that seem out of character), bizarre behavior, and depersonalization. Visual and auditory hallucinations have also been reported. Doral® should be discontinued if these symptoms occur.
Worsening of Depression: Benzodiazepines may worsen depression and consequently, appropriate precautions (e.g., increased monitoring for suicidal ideation, limiting prescription size) should be considered.
ADVERSE REACTIONS
The most common adverse reactions (>1%) observed with DORAL® were drowsiness, headache, fatigue, dizziness, dry mouth, and dyspepsia. Doral® is classified as a Schedule IV controlled substance and patients treated with Doral® should be monitored for tolerance, abuse, and dependence. For a full list of warnings and precautions, please refer to the full prescribing information.
DORAL® contains quazepam, a Schedule IV controlled substance.


